A) 0.370 g/cm3
B) 0.677 g/cm3
C) 1.34 g/cm3
D) 2.70 g/cm3
E) 8.12 g/cm3
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A metal that has a radius of 137 pm crystallizes in a body-centered cubic structure. What is the length of the edge of the unit cell in pm?
A) 137 pm
B) 274 pm
C) 316 pm
D) 387 pm
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of crystal forces hold the units together in solid gold?
A) molecular
B) ionic
C) covalent network
D) metallic bonds
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom sitting in the center position in a body-centered cubic cell is shared among how many cubes?
A) 1
B) 2
C) 3
D) 4
E) 8
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Pick the true statement below.
A) Dipole-dipole forces dominate the intermolecular forces for HBr.
B) The dominant intermolecular force for CH3NH2 is the dispersion force.
C) Hydrogen bonding is the most important intermolecular force for both CH3OH and CHCl3.
D) Hydrogen bonding is the most important intermolecular force for both HF and H2O.
E) All four of these are false.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What intermolecular force(s) of interaction is(are) present between two molecules of dimethyl ether, CH3OCH3? I. London dispersion forces II. Dipole-dipole forces III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What intermolecular force(s) of interaction is(are) possible for a molecule of NH₃shown below?
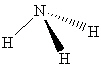
A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) a and b
E) all of these
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules can form hydrogen bonds as a pure liquid?
A) ![]()
B) ![]()
C) ![]()
D) a and b
E) all of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On the basis of intermolecular forces of attraction, rank the following three compounds in terms of increasing boiling point . CH3CH2OH, CH3OCH3 and CH3CH2CH3
A) (lowest bp) CH3CH2OH < CH3OCH3 < CH3CH2CH3 (highest bp)
B) (lowest bp) CH3CH2OH < CH3CH2CH3 < CH3OCH3 (highest bp)
C) (lowest bp) CH3CH2CH3 < CH3OCH3 < CH3CH2OH (highest bp)
D) (lowest bp) CH3CH2CH3 < CH3CH2OH < CH3OCH3 (highest bp)
E) (lowest bp) CH3OCH3 < CH3CH2OH < CH3CH2CH3 (highest bp)
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 11-2 The phase diagram below is needed for the following question(s) . 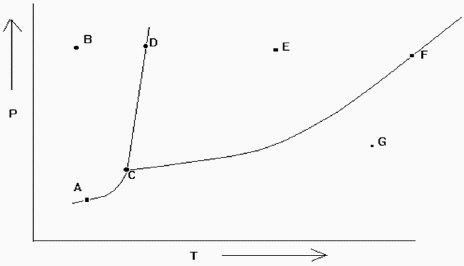 Refer to Exhibit 11-2. At which of the lettered points on the phase diagram is more than one phase present?
Refer to Exhibit 11-2. At which of the lettered points on the phase diagram is more than one phase present?
A) C only
B) A, D, and F only
C) D and F only
D) A, C, D, and F only
E) none of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider liquid state molecules on the submicroscopic scale. Which statement below is true regarding the relative magnitudes of the forces of attraction and forces of disruption among liquid molecules?
A) The forces of attraction are much greater than the kinetic energy for liquid molecules.
B) The forces of attraction and kinetic energy are comparable for liquid molecules.
C) The kinetic energy dominates over the forces of attraction for liquid molecules.
D) A direct comparison between these relative forces cannot be made.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s) . 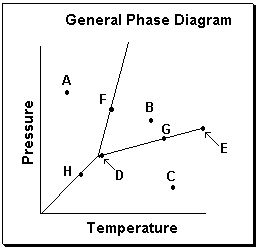 -Refer to Exhibit 11-3. Which temperature-pressure point on this diagram represents conditions in which the solid phase is present in equilibrium with the liquid phase?
-Refer to Exhibit 11-3. Which temperature-pressure point on this diagram represents conditions in which the solid phase is present in equilibrium with the liquid phase?
A) A
B) B
C) C
D) F
E) G
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based upon an analysis of intermolecular forces of attraction among the compounds listed below, which substance would be expected to have the highest boiling point?
A) Br2
B) F2
C) N2
D) H2
E) I2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the three molecules listed below, which choice is best? I. CH3OH II. H2S III. CH3CH3
A) Hydrogen bonding is the dominant intermolecular force only for I.
B) Hydrogen bonding is the dominant intermolecular force for I and II.
C) Hydrogen bonding is the dominant intermolecular force for I, II, and III.
D) Hydrogen bonding is not important for any of these molecules.
E) None of these choices is correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most important intermolecular force in solid H2O?
A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) Ionic bonding
E) Covalent bonding
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances have London Dispersion Forces of attraction? I. He II. CH4 III. H2O
A) I only
B) II only
C) III only
D) I and II
E) All of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A lithium fluoride crystal is classified as:
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a cubic array of atoms, how many unit cells share an atom in the center of a face?
A) 1
B) 2
C) 3
D) 4
E) 8
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the three statements below, pick the best answer. I. Dipole-dipole forces are the main intermolecular force for I - Br. II. O2 is a gas at room temperature because it is highly polarizable. III. London forces dominate the intermolecular forces for Br2.
A) only I is true
B) only II is true
C) only III is true
D) II and III are true, I is false
E) I and III are true, II is false
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the energy of intermolecular attractions are much less than the average kinetic energy of the molecules, what is the physical state of the substance?
A) solid
B) liquid
C) gas
D) plasma
E) Its state cannot be predicted
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 100
Related Exams